Sensory systems are traditionally considered as windows through which the brain perceives the external world. We pose a complementary question:
Can sensory systems serve as windows into brain function and dysfunction?
Among the senses, olfaction is uniquely positioned.
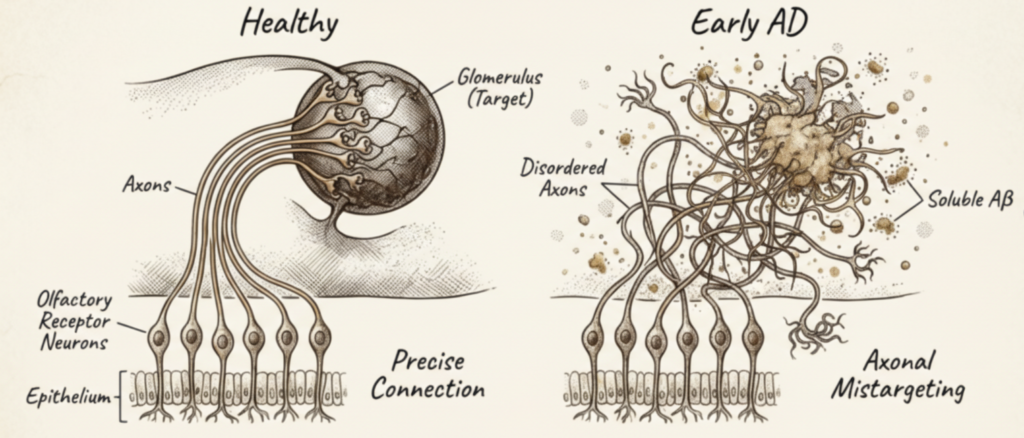
A reduced sense of smell is one of the earliest and most consistent symptoms observed across multiple neurological disorders, including depression and Alzheimer’s disease (AD). Post-mortem studies in AD patients reveal amyloid-beta plaques and neurofibrillary tangles not only in the brain, but also in the olfactory epithelium and olfactory bulb, highlighting early peripheral involvement of the olfactory system.
The glymphatic system and supporting cells: a missing link
Current hypotheses largely overlook the emerging role of the glymphatic system and supporting cells/astrocytes in early neurodegenerative processes.
The glymphatic system is responsible for the clearance of metabolic waste from the brain. Its dysfunction leads to the accumulation of toxic proteins, including amyloid-beta and tau, hallmarks of Alzheimer’s disease.
At the molecular level, this system critically depends on the Aquaporin-4 (AQP4), a water channel expressed by astrocytes and supporting cells.
Importantly, sensory stimulation enhances glymphatic flow, increasing the clearance of amyloid-beta and tau, making the olfactory system a unique interface between sensory activity and brain-wide clearance mechanisms.
Could the nose provide an early warning signal for Alzheimer’s disease?
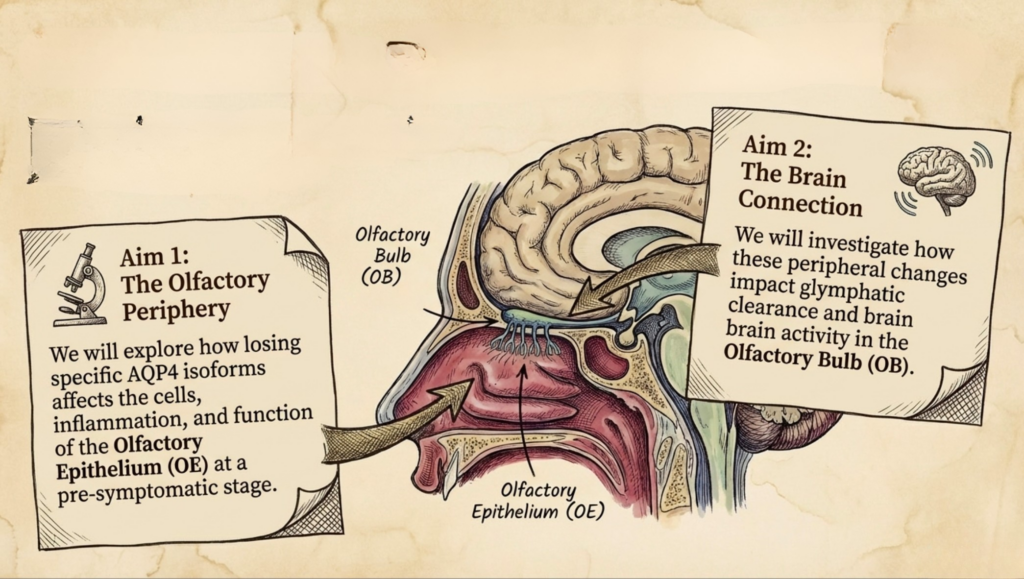
By linking olfactory physiology to glymphatic dysfunction, we aim to uncover previously unexplored mechanisms driving early Alzheimer’s pathology.
Understanding these processes may open new therapeutic avenues, including targeting AQP4 isoforms to enhance waste clearance, with potential applications aimed at slowing disease progression before cognitive symptoms emerge.
